See Full Size
It could be a life-changing device
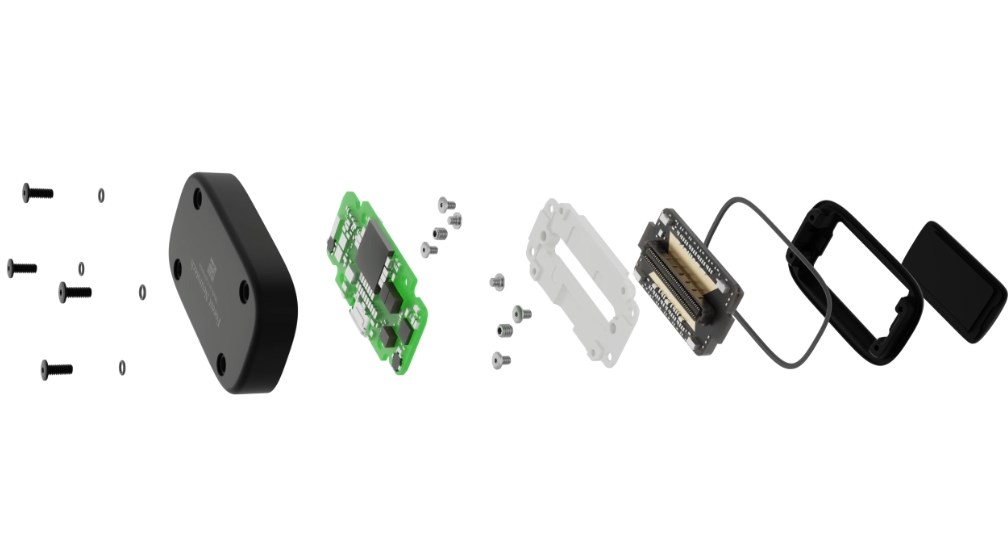
Forest Neurotech developed by and “Forest 1” This device, called Nerve Cells, activates nerve cells in a targeted way by sending ultrasound pulses to certain parts of the brain. The device, an advanced brain-computer interface (BCI), is placed under the skull but outside the brain. Trial, England Agency for Advanced Research and Innovation (Aria) with a budget of £6.5 million and approx. in 30 patients The safety and tolerability of the device will be tested.
See Full Size
How does it work?
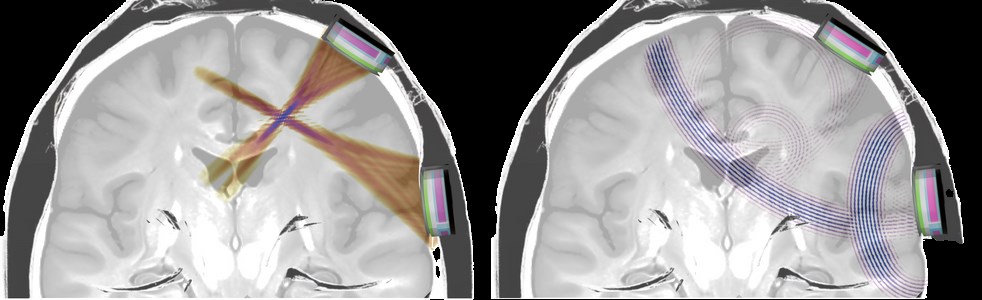
device, ultrasound waves It can create 3D activity maps of the brain by detecting small changes in blood flow. This technology offers approximately 100 times higher resolution than a magnetic resonance imaging (fMRI) device. It can also activate nerve cells in certain brain regions and change emotional states such as motivation or mood. Aria describes the device as “the most advanced BCI in the world” due to its ability to alter activity in multiple regions simultaneously.
See Full Size
Although ultrasound is safe to use, there are safety risks such as the device’s potential to create heat. Prof. from Plymouth University, who took part in the project. Elsa Fouragnan stated that minimizing these risks is a priority. It was also stated that it is of great importance that the device does not undesirably change personality or decision-making processes.
The study will last three and a half years, starting in March, with the first eight months focused on gaining regulatory approval. If successful, Forest will move on to a new full clinical trial for a condition such as depression.
However, important ethical questions are raised during the development and implementation of the device. Clare Elwell, professor of medical physics at University College London (UCL), said: “These innovations may be moving really fast from a technical perspective, but we are lagging behind when it comes to addressing neuroethical issues. “We are now accessing neural pathways in a way that was not possible before, so we need to carefully consider the clinical impact of any intervention and ensure we are always acting in the patient’s best interests.”
Source
https://www.theguardian.com/science/2025/jan/20/brain-implant-boost-mood-ultrasound-nhs-trial
https://forestneurotech.org/our-technology
This news our mobile application Download using
You can read it whenever you want (even offline):